Details of the Drug
General Information of Drug (ID: DMOF7AT)
| Drug Name |
Streptozocin
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Estreptozocina; STREPTOZOTOCIN; STRZ; Streptozocine; Streptozocinium; Streptozocinum; Streptozosin; Zanosar; Alkylating agent; Binds to DNA; Streptozocinium [Latin]; Streptozocine [INN-French]; Streptozocinum [INN-Latin]; Zanosar (TN); Streptozocin (USAN/INN); Streptozocin, Zanosar, STZ,Streptozotocin;N-(Methylnitrosocarbamoyl)-alpha-D-glucosamine; N-D-Glucosyl-(2)-N'-nitrosomethylharnstoff; N-D-Glucosyl-(2)-N'-nitrosomethylurea; D-Glucose, 2-deoxy-2-(((methylnitrosoamino)carbonyl)amino)-(9CI); 1-methyl-1-nitroso-3-[(2S,3R,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]urea; 2-Deoxy-2-(((methylnitrosoamino)carbonyl)amino)-D-glucopyranose; 2-Deoxy-2-(3-methyl-3-nitrosoureido)-D-glucopyranose; 2-Deoxy-2[[(methylnitrosoamino)-carbonyl]amino]-D-glucopyranose; 2-deoxy-2-{[methyl(nitroso)carbamoyl]amino}-alpha-D-glucopyranose
|
|||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
|||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||
| Structure |
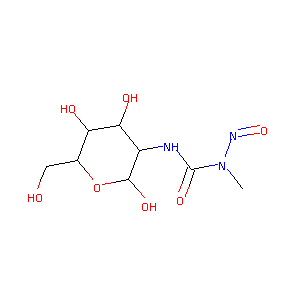 |
|||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 265.22 | ||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.4 | |||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | |||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | |||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Streptozocin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 4 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 5 | Hydrodynamics-based transfection of pancreatic duodenal homeobox 1 DNA improves hyperglycemia and is associated with limited complications in diabe... Endocr J. 2009;56(6):783-90. | ||||
| 6 | Enhanced alpha-kinase 1 accelerates multiple early nephropathies in streptozotocin-induced hyperglycemic mice. Biochim Biophys Acta. 2016 Nov;1862(11):2034-2042. doi: 10.1016/j.bbadis.2016.08.010. Epub 2016 Aug 16. | ||||
| 7 | Impairment of APE1 function enhances cellular sensitivity to clinically relevant alkylators and antimetabolites. Mol Cancer Res. 2009 Jun;7(6):897-906. doi: 10.1158/1541-7786.MCR-08-0519. Epub 2009 May 26. | ||||
| 8 | O(6)-methylguanine DNA-methyltransferase (MGMT) overexpression in melanoma cells induces resistance to nitrosoureas and temozolomide but sensitizes to mitomycin C. Toxicol Appl Pharmacol. 2006 Mar 1;211(2):97-105. doi: 10.1016/j.taap.2005.06.009. Epub 2005 Jul 22. | ||||
| 9 | MnSOD and catalase transgenes demonstrate that protection of islets from oxidative stress does not alter cytokine toxicity. Diabetes. 2005 May;54(5):1437-46. doi: 10.2337/diabetes.54.5.1437. | ||||
| 10 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 11 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 12 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 13 | Bentley ML, Corwin HL, Dasta J "Drug-induced acute kidney injury in the critically ill adult: recognition and prevention strategies." Crit Care Med 38(6 Suppl) (2010): S169-74. [PMID: 20502171] | ||||
| 14 | Product Information. Arava (leflunomide). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 15 | Product Information. Prolia (denosumab). Amgen USA, Thousand Oaks, CA. | ||||
| 16 | Product Information. Clolar (clofarabine). sanofi-aventis, Bridgewater, NJ. | ||||
| 17 | Figg WD, Arlen P, Gulley J, et al. "A randomized phase II trial of docetaxel (taxotere) plus thalidomide in androgen-independent prostate cancer." Semin Oncol 28(4 Suppl 15) (2001): 62-6. [PMID: 11685731] | ||||
| 18 | Product Information. Vumerity (diroximel fumarate). Alkermes, Inc, Cambridge, MA. | ||||
| 19 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 20 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 21 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 22 | Product Information. Arcalyst (rilonacept). Regeneron Pharmaceuticals Inc, Tarrytown, NY. | ||||
| 23 | Product Information. Cimzia (certolizumab). UCB Pharma Inc, Smyrna, GA. | ||||
| 24 | CDC. Centers for Disease Control and Prevention/ "Recommendations of the advisory committtee on immunization practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence." MMWR Morb Mortal Wkly Rep 42(RR-04) (1993): 1-18. [PMID: 20300058] | ||||
| 25 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
